hcl polar or nonpolar
BCl3 is a non-polar molecule then why does it form polar bonds. C 4 H 10 Cl 2 C 4 H 9 Cl HCl.
 |
| Solved Molecule Lewis Structure Total Valence Electrons Chegg Com |
So if I get your sentence right.
. Web Amphiphilic Molecule Some large molecules which have both polar and nonpolar regions. Web Contoh 1. Same with the above reaction when butane reacts. Its hydrophilic water-loving carboxylate group -CO 2 interacts with water molecules via ion-dipole interactions and hydrogen bondingThe hydrophobic water-fearing part of a soap molecule its long nonpolar hydrocarbon chain does not interact with water molecules.
Many compounds are diatomic such as HCl NaCl and KBr. When atoms share an equal number of electrons this sort of covalent connection is produced. Web Types of Covalent Bonds. Web Gugus hidroksil -OH yang terdapat pada alkohol bersifat polar dan hidrofilik tetapi rantai karbonnya bersifat non-polar sehingga hidrofobik.
Diatomic compounds consist of two different elements. Is HCl polar or. Therefore this points to the compound being ionic. The dipole moment of nonpolar molecules is always zero.
Molecules of what type are present in the substance The interaction between solute particles and water molecules which tends to cause a salt to fall apart in water is called Consider two. Additionally which one has the highest boiling point. Web Etanol disebut juga etil alkohol alkohol murni alkohol absolut atau alkohol adalah cairan yang mudah menguap mudah terbakar tak berwarna dan merupakan alkohol yang paling sering digunakan dalam kehidupan sehari-hariSenyawa ini merupakan obat psikoaktif dan dapat ditemukan pada minuman beralkohol dan termometer modern. Important examples include the amino acids and fatty acids.
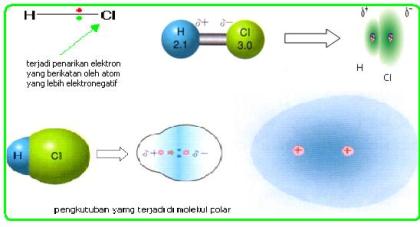
The difference in electronegativity between two atoms is zero. Meskipun atom H dan Cl sama-sama menarik pasangan elektron tetapi keelektronegatifan Cl lebih besar daripada atom H. Web Examples of polar molecules are HCl OF2 etc. Only organic liquids are soluble in non-polar covalent compounds.
Web Reaksi kimia adalah suatu proses alam yang selalu menghasilkan antarubahan senyawa kimia. Rain Drops Are Not Really Drop Shaped. Senyawa kovalen nonpolar terbentuk antara atom-atom unsur yang mempunyai beda keelektronegatifan nol atau. Such as HCl-EtOH solution as solvent.
Polar and Nonpolar 4 Activity. The compound was soluble in water indicating the compound was a polar or ionic compound and not soluble in toluene or acetone which eliminates it being polar or nonpolar. Water and Electrostatic Forces 1 Question Set. Web Hydrolysis is accomplished by treatment of a purified protein with a concentrated acid solution 6N HCl at a very high temperature usually 110 C 230 F for up to 70 hours.
Butane C4H10 is a nonpolar molecule because the difference in electronegativity between carbon255 and hydrogen22 is less than 05 which is way lower to form a polar bond according to the Pauling scale. Polar and Nonpolar 1 Hydrogen Bonds Make Water Sticky 7 Weird Science. 4Cl2 chlorine CCl4 4 HCl acid Before the 1950s the process of manufacturing carbon tetrachloride was done by treating carbon disulfide with chlorine at. No CO 2 is not polar even though the bonds are polar.
Consider the hydrogen chloride HCl molecule. The bond of HCl is also polar just not as much as water. HCl in water for example. In polar fluids polar covalent molecules dissolve.
So In this article I will answer this question and will cover the surrounding topics too. Web Nonpolar molecules usually will dissolve well in nonpolar solvents but tend to be insoluble in water. Examples of Non-polar Molecules Is Carbon Dioxide polar. The polarity of these bonds cancels out making the molecule nonpolar.
Web Di dalam struktur Lewis untuk NaCl dan HCl atom Cl memperoleh konfigurasi elekton atom gas mulia. Water Experiments in Space 1. The boiling point of a system depends upon the intermolecular interactions. Web Remember that polarnonpolar is a continuum.
Web Chemistry students may have doubts about whether CCl4 is polar or nonpolar. Web In organic chemistry a carboxylic acid is an organic acid that contains a carboxyl group COOH attached to an R-group. Web Study with Quizlet and memorize flashcards containing terms like An unknown substance dissolves readily in water but not in benzene a nonpolar solvent. Cl has an electronegativity of 316 and B has 204.
There are many examples too many to list where a polar protic solvent such as water methanol or ethanol can serve as the nucleophile in a reaction often when a. You can check the reason for the polarity of HCl. Web Covalent Bonds. October 28 2013 at 1137 am.
BCl3 is a nonpolar molecule yes and the B-Cl bonds are polar due to the electronegativity difference between the elements. The covalent bond formed by two atoms is said to be nonpolar if the electronegativity of. Web A polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. Web Diatomic molecules consist of two atoms bonded together.
Polar and Nonpolar 1 Further Investigations. Because in these molecules the distribution of charge is always uniform across the entire molecule. Senyawa ataupun senyawa-senyawa awal yang terlibat dalam reaksi disebut sebagai reaktanReaksi kimia biasanya dikarakterisasikan dengan perubahan kimiawi dan akan menghasilkan satu atau lebih produk yang biasanya memiliki ciri-ciri yang berbeda dari. Oxygen as a molecule or O 2 is not a polar molecule because the electronegativities are equal.
Web Is Butane C4H10 polar or non-polar. The polar CCl bonds are oriented 1095 away from each other. Web The unknown compound was then tested for solubility in water toluene and acetone. Air merupakan pelarut universal yang.
Jadi dapat disimpulkan bahwa secara umum senyawa polar larut dalam pelarut polar sedangkan senyawa nonpolar larut dalam pelarut nonpolar. These conditions cleave the peptide bond between each and every amino acid residue. Web Boiling point for the Acids mentioned is 195 C HF -8505 C HCL -66 C HBr and -34 C for HI. The polarity of these bonds cancels out making the molecule nonpolar.
The general formula of a carboxylic acid is RCOOH or RCO 2 H with R referring to the alkyl alkenyl aryl or other groupCarboxylic acids occur widely. In contrast monatomic elements consist of single atoms eg Ar He. The hydrolyzed protein sample is then separated into its constituent amino acids. Web Water H 2 O is a polar inorganic compound that is at room temperature a tasteless and odorless liquid which is nearly colorless apart from an inherent hint of blueIt is by far the most studied chemical compound and is described as the universal solvent and the solvent of life.
In a true covalent bond the electronegativity values are the same eg H 2 O 3 although in practice the electronegativity values just need to be closeIf the electron is shared equally between the atoms forming a covalent bond then the bond is said to be. However because the electronegativities are so high dioxygen can form hydrogen. Web The organic part of natural soap is a negatively-charged polar molecule. The polar CO bonds are oriented 180 away from each other.
Ikatan kovalen polar dapat juga terjadi antara dua atom yang sama tetapi memiliki keelektronegatifan yang berbeda. Web Nonpolar Covalent Bond. Each atom in HCl requires one more electron to form an inert gas electron configuration. In a covalent bond the atoms are bound by shared electrons.
It is the most abundant substance on the surface of Earth and the only. Chlorine has a higher electronegativity than hydrogen but the chlorine atoms attraction for electrons is not. Alkohol dipakai di industri sebagai pelarut atau reagen. As the bond movement formed in BCl3 cancels.
Web The molecule becomes nonpolar with covalent bonds. Because of the linear symmetry of the molecule the negative charges around the oxygen atoms cancel out. Molekulnya secara umum menjadi nonpolar dan semakin tak larut dalam air ketika rantai karbonnya menjadi semakin panjang.
 |
| Polarity Polar Bonds |
 |
| Topic Polarity In Covalent Bonds Do Now What Is The Difference Between A Polar Molecule And Nonpolar Molecule Ppt Download |
 |
| 1 Polarity Polar Bonds Bonds Between Atoms Polar Molecules Polarity Between Molecules Occurs When Polar Bonds Create A Dipole Moment Ppt Download |
 |
| Give Reasons Hcl Is Polar But H2 And Cl2 Are Non Polar |
 |
| Dipole Moment Dielectric Material Polar And Non Polar Molecules |
Posting Komentar untuk "hcl polar or nonpolar"